
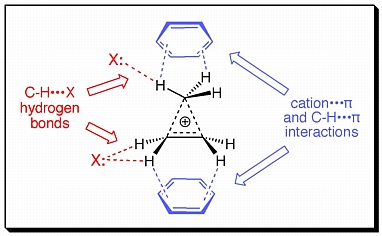
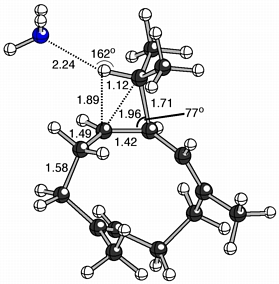
Terpenoid synthases produce a plethora of polycyclic natural products from only a few acyclic precursors. The mechanisms of these impressive enzyme-catalyzed polycyclization reactions have been discussed for decades, but a key unsanswered question is whether or not nonclassical carbocations - carbocations that contain delocalized 3-center 2-electron bonding arrays - are involved in these mechanisms. Nonclassical carbocations are common in nonpolar environments and have even been shown to persist in aqueous solution. It is reasonable, therefore, to think that they may also persist in enzyme active sites. In order to assess the stability of nonclassical carbocations in terpenoid synthase active sites, we are examining complexes of nonclassical species with small models of various protein residues - so-called "theozymes". Some of the types of enzyme-nonclassical cation interactions that are possible are shown schematically above. We have so far shown that complexes between active site residues and intact nonclassical ions can in fact exist, even when the active site residues are quite basic and interact directly with hydrogens whose removal (deprotonation) would lead to cyclopropanes or alkenes. A representative example is shown below.
Hong, Y. J.; Tantillo, D. J., submitted: "Perturbing the Structure of the 2-Norbornyl Cation through C-H---N and C-H---pi Interactions"
Bojin, M. D.; Tantillo, D. J. J. Phys. Chem. A 2006, 110, 4810-481: "Nonclassical Carbocations as C-H Hydrogen Bond Donors"
Gutta, P.; Tantillo, D. J. J. Am. Chem. Soc. 2006, 128, 6172-6179: "Theoretical Studies of Farnesyl Cation Cyclization: Pathways to Pentalenene"
Lodewyk, M. W.; Gutta, P. G.; Tantillo, D. J. J. Org. Chem. 2008, 73, 6570-6579: “Computational Studies on Biosynthetic Carbocation Rearrangements Leading to Sativene, Cyclosativene, alpha-Ylangene, and beta-Ylangene"
Mihaela D. Bojin and Dean J. Tantillo: "Noncovalent Complexes of Nonclassical Cations." Poster presented by Mihaela Bojin at the 229th ACS National Meeting, San Diego, CA, March 13-17, 2005; paper ORGN 895.