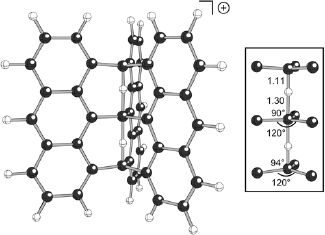
Considerable effort has been expended over the years in search of stable molecules containing carbon atoms in unusual bonding environments. One great triumph in this area was the demonstration that many carbocations contain delocalized 3-center 2-electron bonding arrays. These are the so-called "nonclassical" cations. After pondering whether this sort of delocalization might be extended to larger arrays, and concluding - perhaps naively - that such a situation would be possible, we began a search for a carbocation with a delocalized 5-center 4-electron (C---H---C---H---C)+ bonding array. We explored various helical and polycyclic molecular architectures using B3LYP calculations (uncovering several new 3-center 2-electron cations along the way) and ultimately discovered the extraordinary cation shown above. The central 5-center 4-electron (C---H---C---H---C)+ core of this structure is highlighted to its right, with key bond distances and angles labeled (in Å and degrees, respectively). This remarkable species possesses two trigonal pyramidal carbons flanking a 5-coordinate trigonal bipyramidal carbon! Although only a theoretical molecule at this point, we hope that this unusual carbocation or one of its close relatives might someday be made.
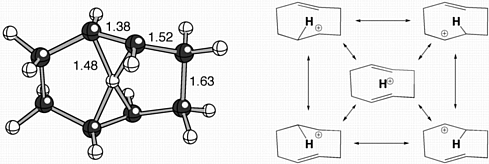
The cations described above contain protons coordinated to two carbon atoms. While this is indeed unusual, and a key feature of many nonclassical cations, the propensity of protons to bond simultaneously to more than two atoms has received comparatively little attention. During our theoretical studies on the mechanisms of terpenoid cyclizations, we discovered a family of unusual cations in which formally 4-coordinate protons are seemingly suspended between two alkenes! A representative "proton sandwich", as we call these species, is shown below, along with various resonance structures that contribute to its overall structure.
Tantillo, D. J.; Hoffmann, R. J. Am. Chem. Soc. 2003, 125, 4042-4043: "Prospecting for a 5-Center 4-Electron (C---H---C---H---C)+ Bonding Array"
Tantillo, D. J.; Hoffmann, R. Angew. Chem. Int. Ed. 2003, 42, 5877-5882: "Breaking Down Barriers: The Liaison Between Sigmatropic Shifts, Electrocyclic Reactions and 3-Center Cations"
Ponec, R.; Yuzhakov, G.; Tantillo, D. J. J. Org. Chem. 2004, 69, 2992-2996: "Multicenter Bonding in Organic Chemistry. Geometry Sensitive 3c-2e Bonding in (C---H---C) Fragments of Organic Cations"
Gutta, P.; Tantillo, D. J. Angew. Chem. Int. Ed. 2005, 44, 2719-2723: "Proton Sandwiches: Nonclassical Carbocations with 4-Coordinate Protons." This article was highlighted in the "Science Concentrates" section of the May 2, 2005 issue of C&EN.
Siebert, M. R.; Tantillo, D. J. J. Org. Chem. 2006, 71, 645-654: "Tetracoordinate Carbon as a Nucleophile? Interconversion of Carbenium Ions with Carbonium Ions Possessing Nearly Square Pyramidal Pentacoordinate Carbons"
Ponec, R.; Bultinck, P.; Gutta, P.; Tantillo, D. J. J. Phys. Chem. A 2006, 110, 3785-3789: "Multicenter Bonding in Carbocations with Tetracoordinate Protons"
Siebert, M. R.; Tantillo, D. J. J. Phys. Org. Chem. 2007, 20, 384-394: "Brother vs. Brother: Competitive Stabilization of Carbocationic Centers Flanked by Cyclopropanes and Alkenes"
Gutta, P.; Tantillo, D. J. Org. Lett. 2007, 9, 1069-1071: "A Promiscuous Proton in Taxadiene Biosynthesis?"
Tantillo, D. J. J. Phys. Org. Chem. 2008, 21, 561-570: "Recent Excursions to the Lands between Concerted and Stepwise: From Natural Products Biosynthesis to Reaction Design"
Lodewyk, M. W.; Gutta, P.; Tantillo, D. J. J. Org. Chem. 2008, 73, 6570-6579: “Computational Studies on Biosynthetic Carbocation Rearrangements Leading to Sativene, Cyclosativene, alpha-Ylangene, and beta-Ylangene"
Wang, S. C.; Tantillo, D. J. Org. Lett. 2008, 10, 4827-4830: "Prediction of a New Pathway to Presilphiperfolanol"
Hong, Y. J.; Tantillo, D. J. J. Am. Chem. Soc. 2009, 131, 7999-8015: "Consequences of Conformational Preorganization in Sesquiterpene Biosynthesis. Theoretical Studies on the Formation of the Bisabolene, Curcumene, Acoradiene, Zizaene, Cedrene, Duprezianene, and Sesquithuriferol Sesquiterpenes"
Tantillo, D. J.; Schleyer, P. v. R. Org. Lett. 2013, 15, 1725-1727: "Nonamethylcyclopentyl Cation Rearrangement Mysteries Solved"
Gutierrez, O.; Harrison, J. G.; Felix, R. J.; Guzman, F. C.; Gagne, M. R.; Tantillo, D. J. Chem. Sci. 2013, 4, 3894-3898: "Carbonium vs. Carbenium Ion-like Transition State Geometries for Carbocation Cyclization - How Strain Associated with Bridging Affects 5-exo vs. 6-endo Selectivity"
Lodewyk, M. L.; Tantillo, D. J. Chirality 2020, 32, 484-488: "Nonclassical Ammonium Ions as Intermediates in Cinchona Alkaloid Rearrangements?," invited for special issue to honor Koji Nakanishi.
Mancinelli, J. P.; Kong, W.-Y.; Guo, W.; Tantillo, D. J.; Wilkerson-Hill, S. M. J. Am. Chem. Soc. 2023, 145, 17389-17397: "Borane-Catalyzed C–F Bond Functionalization of gem-Difluorocyclopropenes Enables the Synthesis of Orphaned Cyclopropanes" (correction: J. Am. Chem. Soc. 2023, 145, 24434-24435)


