Theozymes are theoretical catalysts, constructed by computing the optimal geometry for transition state stabilization by model functional groups. They may be used to quantitate the relative stabilization of reactants and transition states by biological and non-biological catalysts. Theozymes have been used to address many interesting problems in catalysis.
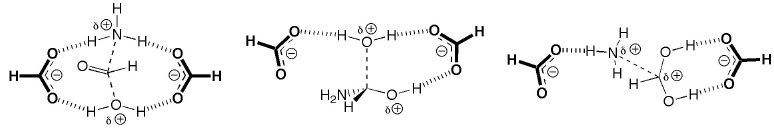
The following picture shows a typical theozyme bound to transition states relevant to peptide hydrolysis by HIV protease. This theozyme (along with many others that have been used to elucidate the mechanisms of various biological catalysts) is discussed in the Current Opinion in Chemical Biology review listed below.
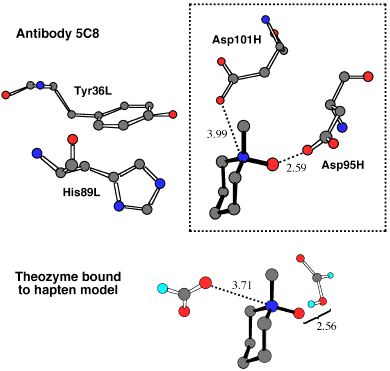
In this next picture, the crystallographically-determined array of functional groups around an ammonium-N-oxide hapten in a catalytic antibody active site is compared to a theozyme predicted to bind tightly to this hapten and the transition states it mimics. This prediction was made years before any structural information was known, yet the congruence between the predicted and observed structures is striking! This demonstration of the potential of theozymes for catalyst design is described in detail in the book chapter listed below.
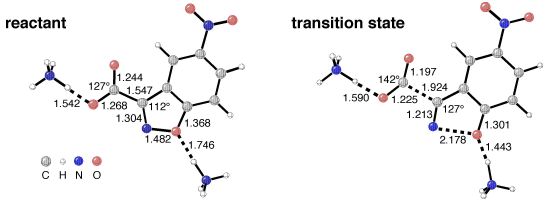
This final picture shows the computed structures of a bis-ammonium theozyme complexed to the reactant and transition state for the decarboxylation of a carboxybenzisoxazole. This recent application of the theozyme technique is described in the third paper listed below.
Tantillo, D. J.; Chen, J.; Houk, K. N. Curr. Op. Chem. Biol. 1998, 2, 743-750: "Theozymes and Compuzymes: Theoretical Models for Biological Catalysis"
Tantillo, D. J.; Houk, K. N.: "Theozymes and Catalyst Design" In Stimulating Concepts in Chemistry, Wiley-VCH: Weinheim, Germany, 2000, pp.79-88.
Ujaque, G.; Tantillo, D. J.; Hu, Y.; Houk, K. N.; Hotta, K.; Hilvert, D. J. Comp. Chem. 2002, 24, 98-110: "Catalysis on the Coastline: Theozyme, Molecular Dynamics, and Free Energy Perturbation Analysis of Antibody 21D8 Catalysis of the Decarboxylation of 5-Nitro-3-Carboxybenzisoxazole," part of a special issue honoring Dr. Peter A. Kollman.
Bojin, M. D.; Tantillo, D. J. J. Phys. Chem. A 2006, 110, 4810-481: "Nonclassical Carbocations as C-H Hydrogen Bond Donors"
Tantillo, D. J. Org. Lett. 2010, 12, 1164-1167: "How an Enzyme Might Accelerate an Intramolecular Diels-Alder Reaction - Theozymes for the Formation of Salvileucalin B"
Bezer, S.; Matsumoto, M.; Lodewyk, M. W.; Lee, S. J.; Tantillo, D. J.; Gagne, M. R.; Waters, M. L. Org. Biomol. Chem. 2014, 12, 1488-1494: "Identification and Optimization of Short Helical Peptides with Novel Reactive Functionality as Catalysts for Acyl Transfer by Reactive Tagging"
Freund, G.; O'Brien, T.; Vinson, L.; Carlin, A.; Yao, A.; Mak, W. S.; Tagkopoulos, I.; Facciotti, M. T.; Tantillo, D. J.; Siegel, J. B. ACS Chem. Biol. 2017, 12, 2465-2473: "Elucidating Substrate Promiscuity within the FabI Enzyme Family"