
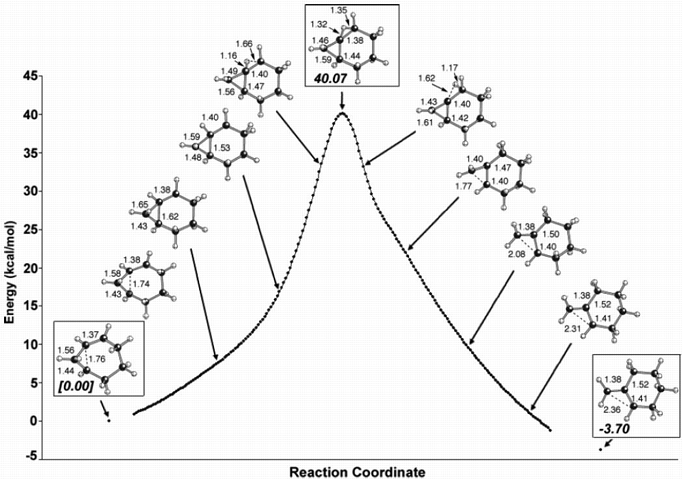
During our studies of carbocation rearrangements we have encountered many reactions that can be described as both concerted and asynchronous. Such reactions involve no intermediates (they are concerted) but they do involve several "events" that do not occur simultaneously (these events occur asynchronously). An example is shown above. The reaction shown is an example of a hiscotropic reaction in which a hydrogen shifts over two carbons and a 3-membered ring opens in a process with a single transition state structure. As you can see from the structures along the reaction coordinate, however, migration of the hydrogen precedes ring-opening. Such processes are sometimes tied to potential energy surfaces with unsusual features such as flat plateaues and bifurcations. We have found that concerted asynchronous reactions may also be common occurrences in the processes used by Nature to construct complex terpene natural products. Key papers:
Nouri, D. H.; Tantillo, D. J. J. Org. Chem. 2006, 71, 3686-3695: "Hiscotropic Rearrangements: Hybrids of Electrocyclic and Sigmatropic Reactions"
Tantillo, D. J. J. Phys. Org. Chem. 2008, 21, 561-570: "Recent Excursions to the Lands between Concerted and Stepwise: From Natural Products Biosynthesis to Reaction Design"
Tantillo, D. J. Chem. Soc. Rev. 2010, 39, 2847-2854: "The Carbocation Continuum in Terpene Biosynthesis - Where are the Secondary Cations?" invited tutorial review.
Tantillo, D. J. Nat. Prod. Rep. 2011, 28, 1035-1053: "Biosynthesis via Carbocations: Theoretical Studies on Terpene Formation"
Hong, Y. J.; Ponec, R.; Tantillo, D. J. J. Phys. Chem. A 2012, 116, 8902-8909: "Changes in Charge Distribution, Molecular Volume, Accessible Surface Area and Electronic Structure Along the Reaction Coordinate for a Carbocationic Triple Shift Rearrangement of Relevance to Diterpene Biosynthesis"
Ortega, D. E.; Tantillo, D. J.; Toro-Labbe, A. Phys. Chem. Chem. Phys. 2015, 17, 9771-9779: "Detailed Analysis of the Mechanism of a Carbocationic Triple Shift Rearrangement"
back to list of research projects