"Hydrogen is a light, odorless gas, which, given enough time, turns into people."
- Edward R. Harrison, Astronomer/Cosmologist
"For first the first-beginnings of things move of themselves; then those bodies which are formed of a tiny union, and are, as it were, nearest to the powers of the first-beginnings, are smitten and stirred by their unseen blows, and they in their turn, rouse up bodies a little larger. And so the movement passes upwards from the first-beginnings, and little by little comes forth to our senses, so that those bodies move too, which we can descry in the sun’s light."
- Titus Lucretius Carus, "On the Nature of Things"
"...there are things that are not absolutely correct with a capital 'C', but extremely worthwhile because they're major assumptions which allow things to move forward."
- Gilbert Stork, Chemical Heritage Foundation Oral History
"'Perfect' means you get to a point where there's nowhere left to go."
- Natasha Vita-More, SYFY25 Origin Stories podcast
"Our goal is not truth, a philosophical term, but rather a consistent description and interpretation of phenomena within a model concept."
- Rof Huisgen, The Adventure Playground of Mechanisms and Novel Reactions
"The only criterion of a model is its usefulness, not its 'truth'."
- Michael Dewar, J. Am. Chem. Soc. 1984, 106, 669
"...the search for fact, not truth"
- Dr. Henry Jones, Jr., "Indiana Jones and the Last Crusade"
"Chemistry needs models but chemistry also needs to recognize that models are models."
- Timothy Clark and Martin G. Hicks, "Models of Necessity"

Fellow, American Chemical Society (ACSF), 2017
Fellow, Royal Society of Chemistry (FRSC), 2016
Fellow, American Association for the Advancement of Science (AAAS), 2016
Professor, UC Davis, 2011-present
Associate Professor, UC Davis, 2008-2011
Assistant Professor, UC Davis, 2003-2008
Postdoctoral Associate, Cornell University, 2000-2003
PhD, Chemistry, UCLA, 1995-2000
AB, Chemistry, Harvard University, 1991-1995
Born (1973) and raised in Quincy, Massachusetts, USA
Ramblings...
on why I like being a professor
on drawing structures and air hockey
The Tantillo group is committed to cultivating an inclusive laboratory by respecting and engaging all members, providing equitable access to opportunities and resources, and embracing new perspectives and ideas. We believe that diversity in knowledge, life experiences, self-expression, and identity furthers chemistry knowledge and enriches the chemistry community. We value the uniqueness of each member, because our differences are our greatest strength.
![]()
Our research is driven by intriguing mechanistic and structural questions and spans many areas of organic chemistry. These include natural products synthesis and biosynthesis, enzyme catalyzed reactions, polycyclization reactions, catalyst design, physical organometallic chemistry, carbocation structures and rearrangements, pericyclic reactions, regio- and stereoselectivity of synthetically useful reactions, and computer-aided design of enzyme inhibitors and unusual substrates.
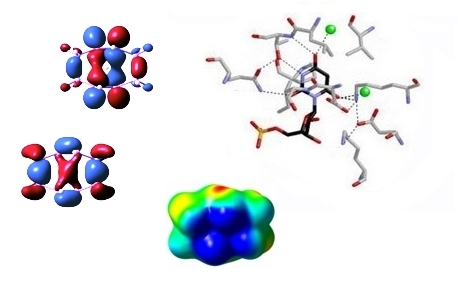
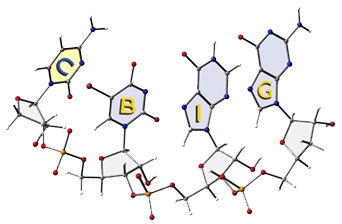
Our group is particularly intrigued by the mechanisms of carbocation cyclization/rearrangements used by Nature to synthesize complex terpene natural products such as those shown below.
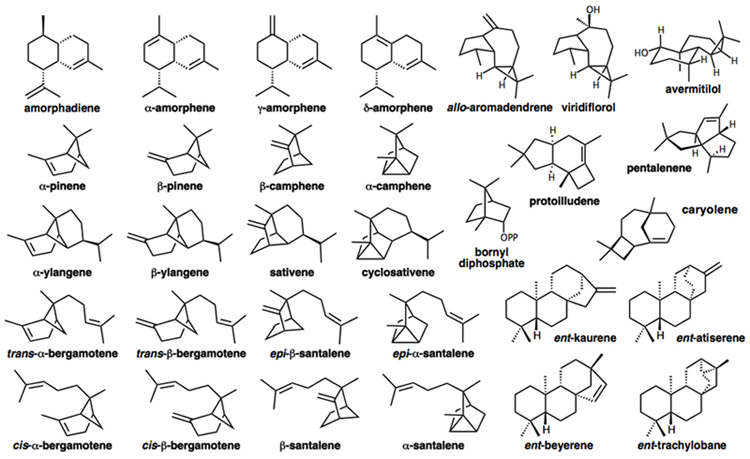
Our primary tool for tackling mechanistic problems is quantum chemistry. We apply various methods - e.g., density functional theory (DFT) - to compute structures, relative energies, activation barriers, potential energy surface (PES) shapes, NMR/IR/UV/VCD spectra, isotope effects, and solvation effects. We also make use of ab initio direct dynamics trajectory calculations to explore nonstatistical dynamic effects on reactivity.
Using these methods, we like to make structural and reactivity predictions and have these predictions put to the test in the wet lab.
Reactivity/selectivity models and methods we have helped develop:
general
• extreme asynchrony in circumventing orbital symmetry constraints (Tetrahedron 2008, 64, 5672)
• transition state complexation (e.g., J. Am. Chem. Soc. 2007, 129, 8686)
• inter-transition state roaming (e.g., J. Am. Chem. Soc. 2021,143, 1088)
• dynamic mismatching (e.g., J. Am. Chem. Soc. 2024, 146, 7039)
• sulfur-lone pair interactions are not necessarily dominated by lone pair donation to S–X antibonding orbitals (e.g., Org. Biomol. Chem. 2017, 15, 3179)
• analogies between photochemical reactions and ground state reactions with post-transition state bifurcations (e.g., Nature Chem. 2024, 16, 615)
portable models of entropy
• a PES perspective: width, not just height, of pathways to products matters (e.g., Chem. Sci. 2020, 11, 9937)
• a non-covalent interaction perspective: electrostatic drag (e.g., Phys. Chem. Chem. Phys. 2020, 22, 26955)
selective synthetic reactions
• post-transition state bifurcations as sources of side products (e.g., Chem. Sci. 2017, 8, 1442)
• modulating selectivity for reactions with post-transition state bifurcations using non-covalent interactions (e.g., J. Am. Chem. Soc. 2017, 139, 7485)
• modulating selectivity for carbocation rearrnagements using non-covalent interactions (e.g., Nature 2024, 625, 287)
• catalyst dissociation before catalytic cycle closure (e.g., J. Am. Chem. Soc. 2016, 138, 487)
• circumventing the Woodward-Hoffmann rules by design (e.g., Angew. Chem. Int. Ed. 2023, 62, e202300288)
natural products biosynthesis
• dominance of inherent substrate reactivity in some enzymatic reactions (e.g., Angew. Chem. Int. Ed. 2017, 56, 10040)
• post-transition state bifurcations as selectivity control elements in natural product biosynthesis (e.g., Nature Chem. 2014, 6, 104)
• dyotropic and triple-shift reactions increase the efficiency of terpene formation in Nature (e.g., J. Org. Chem. 2018, 83, 3780)
• secondary carbocations are rarely minima (e.g., Chem. Soc. Rev. 2010, 39, 2847)
• TerDockin, a tool for docking carbocations into terpene synthases (e.g., ACS Catal. 2018, 8, 3322)
• theory as an inspiration for natural product total synthesis (e.g., J. Am. Chem. Soc. 2012, 134, 18550 → Angew. Chem. Int. Ed. 2019, 58, 9851)
CheShiReCCat Chemical Shift/Coupling Constant Scaling Factor Website
Events
Theoretical Physical Organic Chemistry (TPOC) Meetings ('21ff)
Accelerating Reaction Discovery TSRC Workshop ('11ff)
West Coast Theoretical Chemistry Meeting ('23)
45th National Organic Symposium ('17)
Walking in the Woods with Chemistry at the UCD Arboretum ('16)
Groups
UCD Chemical Biology Program (CBP)
ACS Chemists with Disabilities
Alliance for Diversity in Science and Engineering (UCD Chapter)
Agricultural and Environmental Chemistry Graduate Group
![]()
Dean has taught both graduate and undergraduate chemistry classes for both chemistry and non-chemistry audiences. These classes have ranged in size from less than ten to over three hundred students. For more details, follow the links below.
Dean's Teaching Experience and Awards
Databank of Dynamics Trajectories (DDT)
Pharmaceutical Chemistry Undergraduate Program
![]()
Awards Earned by Group Members
Representation and presentation
Old books and physical organic history


![]()
Contact
Dean  with any questions, comments, or suggestions...
with any questions, comments, or suggestions...