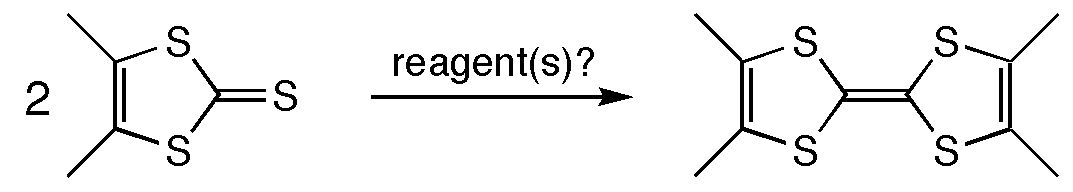
Tetrathiafulvalenes(TTFs) have received considerable attention due to their ability to serve as electron donors in charge transfer salts which behave as "organic metals." This project, undertaken during a brief stay in the group of Craig Merlic, involved the development of strategies for efficient synthesis of tetramethyltetrathiafulvalene (TMTTF) from 4,5-dimethyl-1,3-dithiol-2-thione in order to provide the research group of Stuart Brown in the Department of Physics and Astronomy at UCLA with large quantities of material for solid state NMR studies. Standard methodologies for coupling 1,3-dithiol-2-thiones to obtain tetrathiafulvalenes failed for this substrate. Novel methods such as McMurry coupling of 4,5-dimethyl-1,3-dithiol-2-thione and potassium-induced dimerization via the 4,5-dimethyl-1,3-dithiol-2-ylidene were also unsuccessful. Direct coupling by the action of dicobaltoctacarbonyl proceeded in modest yield (up to ~60%) and proved to be the only practical route to TMTTF. Once the protocol for TMTTF synthesis was optimized, gram-scale quantities of TMTTF and a doubly 13C-labeled TMTTF were synthesized. The Brown group continues to examine the magnetic properties of charge transfer salts derived from labeled and unlabeled TMTTF.
Chow, D. S.; Wzietek, P.; Fogliatti, D.; Alavi, B.; Tantillo, D. J.; Merlic, C. A.; Brown, S. E. Phys. Rev. Lett. 1998, 81, 3984-3987: "Singular Behavior in the Pressure-Tuned Competition between Spin-Peierls and Antiferromagnetic Ground States of (TMTTF)2PF6"
Brown, S. E.; Clark, W. G.; Alavi, B.; Chow, D. S.; Merlic, C. A.; Tantillo, D. J. Synthetic Metals 1999, 103, 2056-2057: "Incommensurate Phase of the Organic Spin-Peierls Compound (TMTTF)2PF6"
Merlic, C. A.; Baur, A.; Tantillo, D. J.; Brown, S. E. Synth. Commun. 1999, 29, 2953-2958: "Synthesis of Carbon-13 Labeled Tetramethyltetrathiafulvalene"
Brown, S. E.; Clark, W. G.; Alavi, B.; Hall, D.; Naughton, M. J.; Tantillo, D. J.; Merlic, C. A. Phys. Rev. B. 1999, 60, 6270-6272: "High-field Magnetization of the Spin-Peierls Compound (TMTTF)2PF6"
Chow, D. S.; Zamborszky, F.; Alavi, B.; Tantillo, D. J.; Baur, A.; Merlic, C. A.; Brown, S. E. Phys. Rev. Lett. 2000, 85, 1698-1701: "Charge Ordering in the TMTTF Family of Molecular Conductors"