
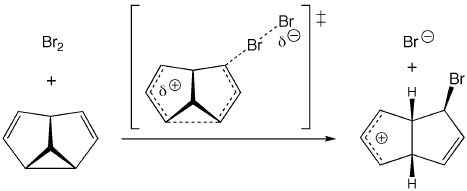
The bromination of semibullvalene was reported decades ago by Paquette and coworkers. We have revisted this reaction, subjecting it to analysis by modern computational quantum chemistry. Of the various possible mechanisms for bromination - formation of a bridged bromonium cation and subsequent ring-opening, formation of a cyclopropylcarbinyl cation and subsequent ring-opening, concerted bromine addition and ring-opening - we find that the concerted process is energetically favored. This process (shown above) leads directly to an allyl cation and releases ring strain. We have also modeled the regio- and selectivity of incorporation of both the first bromine atom (formally incorporated as Br+) and the second bromine atom (incorporated as Br-); the predictions from these calculations agree with the experimentally determined selectivity.
Wang, S. C. Tantillo, D. J. Eur. J. Org. Chem. 2006, 738-745: "The Mechanism of Semibullvalene Bromination"
Wang, S. C..; Tantillo, D. J. J. Phys. Chem. A 2007, 111, 7149-7153: "Selective Stabilization of Transition State Structures for Cope Rearrangements of Semibullvalene and Barbaralane through Interactions with Halogens"
Selina C. Wang and Dean J. Tantillo: "Reactions of Semibullvalene and Its Derivatives." Poster presented by Selina Wang at the Bradford Borge Weekend, University of California, Davis, CA, February 25-26, 2005.