
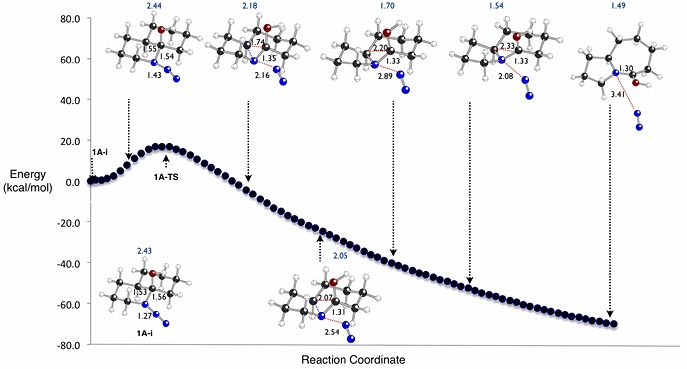
In collaboration with the Aube group at the University of Kansas, we have used density functional theory calculations to explore the mechanisms of Schmidt rearrangements like the one shown above. This reaction was found to proceed through rapid formation of an azidohydrin intermediate (1A-i), followed by rate-determining concerted N2-loss/shift of the alkyl group antiperiplanar to the N2 leaving group. For related systems where steric, lone pair-cation and cation-pi effects have been invoked as regiocontrol elements, the origins and magnitudes of these effects have been examined theoretically.
Gutierrez, O.; Aube, J.; Tantillo, D. J. J. Org. Chem. 2012, 77, 640-647: "Mechanism of the Acid Promoted Intramolecular Schmidt Reaction. Theoretical Assessment of the Importance of Lone Pair-Cation, Cation-pi and Steric Effects in Controlling Regioselectivity"