What motivations shape how a scientist writes a paper? How important is it to let the reader know the order of events that led to the results reported therein?
Consider these two versions of a paper I wrote on some calculations I performed just after the arrival of the new millennium...
A Theoretical Study of NO2PS2
Dean J. Tantillo [1]
Department of Chemistry and Chemical Biology Cornell University Ithaca, New York 14853-1301
Abstract. Quantum mechanical calculations were performed on an isomer of NO2PS2 that is analogous to ONOONO. Important differences between the trans-perp-trans isomers of ONOONO and NO2PS2 were discovered, and their origins and implications are discussed.
The many isomers of N2O4 have been the subject of extensive study due to their importance in the chemistry of the troposphere and stratosphere.[2,3] To the best of our knowledge, higher molecular weight analogs of N2O4 have not been studied. Herein we describe quantum mechanical calculations on the structure and vibrational properties of the trans-perp-trans isomer of NO2PS2, an analog of linear ONOONO. Hopefully, this comparison of N2O4 and NO2PS2 will facilitate understanding of the unique and environmentally-relevant properties of the former.
The geometry and vibrational spectrum of the trans-perp-trans isomer of NO2PS2 were computed using second order Moller-Plesset perturbation theory (MP2) and a double-zeta quality basis set (6-31G*). This same method was used recently to study isomers of N2O4,[2] thereby facilitating a direct comparison of our computational results with those reported for ONOONO.
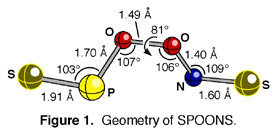
The computed geometry of the trans-perp-trans conformational isomer of NO2PS2 is shown in Figure 1. Interestingly, the central O-O bond of NO2PS2 is considerably longer (by 0.05 A), and the internal O-N bond is notably shorter (by 0.13 A), than those in ONOONO.[2] The is perhaps a result of the enhanced electron-accepting ability of the N=S fragment, and indicates that O-N bond cleavage in NO2PS2 to produce NS may be more difficult than O-N bond cleavage in ONOONO to produce the atmospherically and biologically important NO free radical.
Much to our satisfaction, the predicted vibrational modes for NO2PS2 mirror those reported for ONOONO, despite the lower symmetry of NO2PS2. For example, the highest intensity mode for trans-perp-trans NO2PS2 is predicted to occur at 743 cm-1 and is dominated by P-O and O-N stretching, while the highest intensity mode for trans-perp-trans ONOONO occurs at 736 cm-1 and corresponds to N-O stretching.[2]
In conclusion, we have predicted the preferred geometry and vibrational spectrum of the novel N2O4 analog NO2PS2. This information will facilitate our understanding of the chemistry of such molecules, and provides a stepping stone for further studies on additional molecules of this type. Efforts in this direction are in progress, and will be reported in due course.
(1) This work was supported by the Institute. We acknowledge Dr. Bruce Hietbrink for many helpful insights.
(2) Wang, X.; Qin, Q-Z.; Fan, K. J. Mol. Struc. (Theochem) 1998, 432, 55-62 and references therein.
(3) Olson, L. P.; Kuwata, K. T.; Bartberger, M. D.; Houk, K. N. J. Am. Chem. Soc. 2002, 124, 9469-9475.
On Bending SPOONS
Dean J. Tantillo [1]
Department of Chemistry and Chemical Biology Cornell University Ithaca, New York 14853-1301
Abstract. Quantum mechanical calculations were performed on SPOONS that show that there is not an inherent tendency for SPOONS to bend.
Herein we (all one of us) describe expensive quantum mechanical calculations on a molecule of no relevance to the real world. This molecule is one of several isomers bearing the empirical formula NO2PS2. The connectivity of the particular isomer with which we are concerned can - in fact should - be denoted as SPOONS. Although SPOONS is actually analogous to molecules important in atmospheric chemistry (such as the N2O4 isomer ONOONO),[2,3] it is the subject of this paper solely because we first thought of the title. While we are among the chemical community's biggest supporters of (1) investigations into cool-molecules-with-no-practical-utility and (2) title-driven project design, even we find it difficult to admit that time and resources were actually devoted to these calculations.
In any case, we did in fact calculate the geometry and vibrational spectrum of SPOONS using second order Moller-Plesset perturbation theory (MP2) and a double-zeta quality basis set of much renown (6-31G*). This same method was used recently to study ONOONO,[2] thereby allowing us to compare our results directly with those reported for ONOONO, although we don't really know what that will show.
The computed geometry of the trans-perp-trans isomer of SPOONS is shown in Figure 1. We had hoped to report that the bond distances, bond angles, and dihedral angles in this structure are unremarkable. Unfortunately, the central O-O bond is a bit long, and the internal O-N bond is much shorter than in ONOONO.[2] If any readers would like us to explain these unexpected observations, you may write to us at the address above - please indicate whether you would prefer a photograph or sketch of waving hands.

Much to our dissatisfaction, dismay and chagrin, almost none of the predicted vibrational modes for SPOONS actually correspond to bending. In fact, only the vibrational mode at 335 cm-1 shows a significant bending component (that for O-N-S bending), and the intensity of this mode is so small that we have little hope that it might be detected experimentally. In contrast, the highest intensity modes have large components corresponding to P-O, O-O, and O-N stretching. Damn.
In conclusion, we have actually shown very little. We can say, however, that we observe no inherent tendency of SPOONS to bend, suggesting that such a process would indeed require focused external intervention, be it telekinetic or otherwise.
(1) This work was supported by the Institute. We acknowledge Dr. Bruce Hietbrink because he enjoys being acknowledged.
(2) Wang, X.; Qin, Q-Z.; Fan, K. J. Mol. Struc. (Theochem) 1998, 432, 55-62 and references therein.
(3) Olson, L. P.; Kuwata, K. T.; Bartberger, M. D.; Houk, K. N. J. Am. Chem. Soc. 2002, 124, 9469-9475.