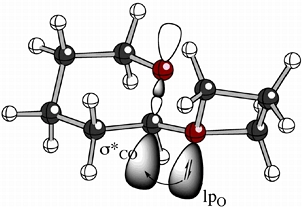
We are exploring the electronic structures and reactions of unusual oxonium ions, i.e. cations containing 3-coordinate oxygen atoms.
Among the oxonium ions we have studied are oxiranium (epoxonium) and oxetanium ions formed in the reactions of cyclic ethers with glycosyl iodides - reactions discovered by our collaborators in the Gervay-Hague group at UC Davis. An example of a small model of such systems is shown above. Overlaid on the ball & stick structure of this oxetanium ion are cartoons showing the interaction of the oxygen lone pair on the oxetanium ion with the antibonding orbital of the C-O bond in the 6-membered ring (a simple model of a sugar), an interaction that causes the rotamer shown to be preferred for such systems. This is an example of an "exo-anomeric effect".
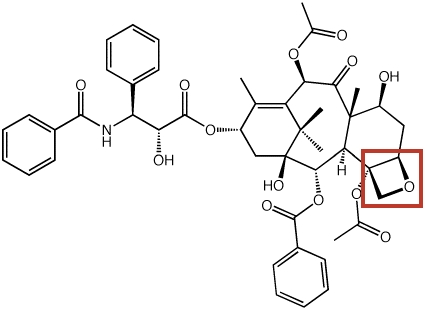
We have also examined polycyclic oxabicyclobutonium ions that may be involved in the biosynthesis of the oxetane ring in the anti-cancer agent taxol (below), a diterpenoid natural product.
El-Badri, M. H.; Willenbring, D.; Tantillo, D. J.; Gervay-Hague, J., J. Org. Chem. 2007, 72, 4663-4672: "Mechanistic Studies on the Stereoselective Formation of beta-Mannosides from Mannosyl Iodides Using alpha-Deuterium Kinetic Isotope Efffects"
Willenbring, D.; Tantillo, D. J. Russ. J. Gen. Chem. 2008, 78, 723-731 (Rossiiskii Khimicheskii Zhurnal 2007, 51, 49-55): "Mechanistic Possibilities for Oxetane Formation in the Biosynthesis of Taxol's D Ring"
Dan Willenbring and Dean J. Tantillo: "Mechanistic Possibilities for Oxetane Formation in the Biosynthesis of Taxol's D Ring." Poster presented by Dan Willenbring at the SYLICCO.07 Symposium, Davis, CA, July 26, 2007.