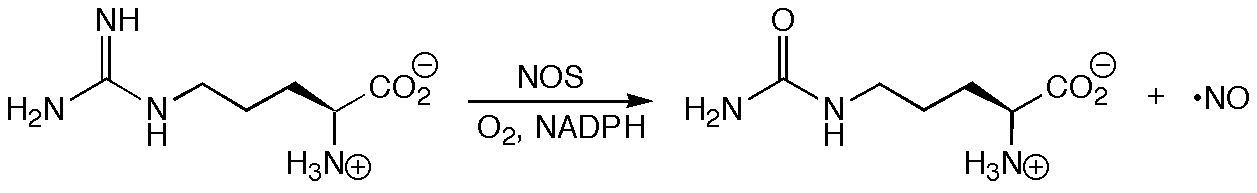
In light of its role as an intermediate in the biosynthesis of the important biological messenger nitric oxide (NO), N-hydroxyarginine (NOHA) has been studied extensively by the groups of Jon Fukuto in the Department of Pharmacology at UCLA and Richard Silverman in the Departments of Chemistry and Biochemistry, Molecular Biology, and Cell Biology at Northwestern University.
The details of the mechanism by which Nitric Oxide Synthase (NOS) converts arginine to NOHA and then NOHA to citrulline and NO remain elusive. Among the many issues needing resolution are (a) which form of NOHA (i.e. protonation state and tautomer) is bound to NOS, and (b) which radical species are derived from NOHA on the pathway to NO. Computations using density functional theory, in combination with structural data derived from X-ray and ENDOR experiments, suggest that bound NOHA is protonated and that a radical derived by hydrogen atom abstraction from nitrogen is a plausible alternative to the O-radicals that are usually invoked. Recent studies on the structure of NOS-NOHA complexes by Crane and Tainer and on the reactivity of NOHA analogues by Silverman suggest that a mechanism involving N-radical formation is indeed viable.
Surprisingly, it has also been shown that under certain conditions NOHA is released from NOS in large quantities rather than being further processed to NO. The physiological role of NOHA is unclear, but studies in the Fukuto group suggest that, through reactions with oxygen or NO itself, NOHA may function as a biological carrier of NO or nitroxyl (HNO) (which the Fukuto group has shown to be a potent vasorelaxant). In order to rationalize known experimental data and to guide further experiments, conformational, spectroscopic and redox properties of NOHA were examined computationally. Theory was also used to investigate the possible reactions between NOHA and NO. Preliminary results suggest that electron transfer processes may initiate reaction between NOHA and NO, leading to species that may survive long enough to diffuse considerable distances before decomposing to release HNO.
Tantillo, D. J.; Fukuto, J. M.; Hoffman, B. M.; Silverman, R. B.; Houk, K. N. J. Am. Chem. Soc. 2000, 122, 536-537: "Theoretical Studies on NG-Hydroxy-L-arginine and Derived Radicals: Implications for the Mechanism of Nitric Oxide Synthase"