
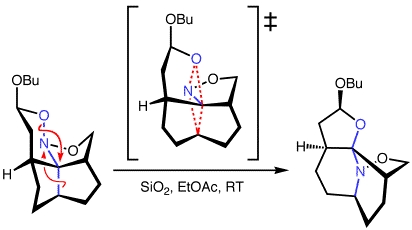
We have used density functional theory calculations to explore the mechanisms of dyotropic rearrangements like the nitroso acetal-to-aminal rearrangements recently reported by Denmark and coworkers (above). Our calculations indicate that these sorts of reactions are indeed concerted dyotropic rearrangements. We have also explored the effects of various structural and environmental factors on the barriers for rearrangement, showing that the ease of these reactions results from both the structural constraints imposed by the molecule and selective transition state stabilization by a polar protic environment.
We have encountered related processes in terpene biosynthesis.
Davis, R. L.; Tantillo, D. J. J. Org. Chem. 2010, 75, 1693-1700: "Dissecting a Dyotropic Rearrangement"
Davis, R. L.; Leverett, C. A.; Romo, D.; Tantillo, D. J. J. Org. Chem. 2011, 76, 7167-7174: "Switching Between Concerted and Stepwise Mechanisms for Dyotropic Rearrangements of beta-Lactones Leading to Spirocyclic, Bridged gamma-Butyrolactones"
Leverett, C. A.; Purohit, V. C.; Johnson, A. G.; Davis, R. A.; Tantillo, D. J.; Romo, D. J. Am. Chem. Soc. 2012, 134, 13348-13356: "Dyotropic Rearrangements of Fused Tricyclic-beta-Lactones: Application to the Synthesis of (-)-Curcumanolide A and (-)-Curcumalactone"
Gutierrez, O.; Tantillo, D. J. J. Org. Chem. 2012, 77, 8845-8850: "Analogies Between Synthetic and Biosynthetic Reactions in which [1,2]-Alkyl Shifts are Combined with Other Events - Dyotropic, Schmidt and Carbocation Rearrangements," invited JOCSynopsis.
Hashimoto, Y.; Kong, W.-Y.; Tantillo, D. J. Org. Lett. 2024, 26, 5441-5446: "Discovery of a Formal Dyotropic Rearrangement during Acid-mediated Dioxabicyclo[4.2.1]nonanone Formation"
Xu, B.; Zhang, Z.; Tantillo, D. J.; Dai, M. J. Am. Chem. Soc. 2024, 146, 21250-21256: "Concise Total Syntheses of (–)-Crinipellins A and B Enabled by a Controlled Cargill Rearrangement"
Rebecca L. Davis and Dean J. Tantillo: "Investigation into the Dyotropic Rearrangement of a Nitroso Acetal." Poster presented by Rebecca Davis at the SYLICCO.07 Symposium, Davis, CA, July 26, 2007.
Rebecca L. Davis and Dean J. Tantillo: "Investigation into the Dyotropic Rearrangement of a Nitroso Acetal." Poster presented by Rebecca Davis at the Bradford Borge Weekend, University of California, Davis, CA, February 21-22, 2009.
Rebecca L. Davis and Dean J. Tantillo: "Investigation into the Dyotropic Rearrangement of a Nitroso Acetal." Poster presented by Rebecca Davis at Department of Chemistry Recruiting Weekend Poster Session, University of California, Davis, CA, March 19-20, 2009.
Rebecca L. Davis and Dean J. Tantillo: "Mechanistic Studies on the Dyotropic Rearrangement of Tricyclic Spirolactones." Poster presented by Rebecca Davis at the R. Bryan Miller Symposium, UC Davis, Davis, CA, April 2-3, 2009.
Rebecca L. Davis and Dean J. Tantillo: "Mechanistic Studies on the Dyotropic Rearrangement of Tricyclic Spirolactones." Poster presented by Rebecca Davis at the National Organic Symposium, Boulder, CO, June 7-11, 2009; paper D31.
Rebecca L. Davis and Dean J. Tantillo: "Theoretical Study of Dyotropic Rearrangements Involving a Migrating Oxygen." Lecture presented by Rebecca Davis at the 239th ACS National Meeting, San Francisco, CA, March 21-25, 2010; paper ORGN 108.
Dean J. Tantillo: "Dissecting Dyotropic Reactions: Theoretical Studies of Synthetic and Biosynthetic Rearrangements." Invited lecture presented at NovaBay Pharmaceuticals, Emeryville, CA, April 21, 2010.