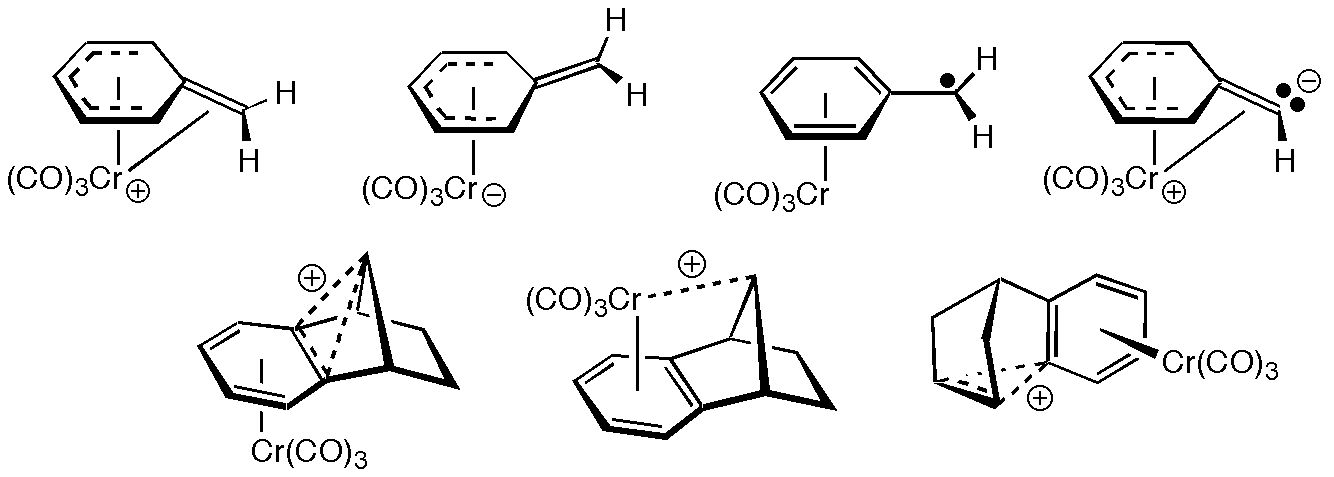
It is known that both benzyl cations and anions are stabilized by Cr(CO)3 complexation. This unusual characteristic of chromium arenes has been the subject of many synthetic, spectroscopic and physical organic studies. The effect of Cr(CO)3 on benzylic radicals has received comparatively little attention, however. Density functional theory calculations, initiated in response to experiments performed in the laboratory of Craig Merlic in the Department of Chemistry and Biochemistry at UCLA, demonstrate that direct interaction between chromium and the benzylic position is present in Cr(CO)3-complexes of benzyl cations but not in the analogous complexes of anions and radicals, as shown above. Strengthened orbital interactions in the ions lead to enhanced stability and are accompanied by delocalization of charge from the benzyl ligands to the Cr(CO)3 fragment. Theory predicts that similar effects should control the strucure and reactivity of Cr(CO)3-complexed singlet aryl carbenes, as well as Cr(CO)3 complexes of homobenzylic reactive intermediates (many of which have nonclassical structures in the absence of chromium).
Merlic, C. A.; Walsh, J. C.; Tantillo, D. J.; Houk, K. N. J. Am. Chem. Soc. 1999, 121, 3596-3606: "Chemical Hermaphroditism: The Potential of the Cr(CO)3 Moiety to Stabilize Transition States and Intermediates with Anionic, Cationic or Radical Character at the Benzylic Position"
Tantillo, D. J.; Hietbrink, B. N.; Merlic, C. A.; Houk, K. N. J. Am. Chem. Soc. 2000, 122, 7136-7137 (additional note: J. Am. Chem. Soc. 2001, 123, 5851): "Attenuating and Supplanting Nonclassical Stabilization: Cr(CO)3-Complexed Benzonorbornenyl Cations"
Hietbrink, B. N.; Tantillo, D. J.; Houk, K. N.; Merlic, C. A.: "Chromium Tricarbonyl Coordinated Carbocations" In Carbocation Chemistry, Wiley Interscience: Hoboken, NJ, 2004, pp. 279-289.
Gribanova, T. N.; Starikov, A. G.; Minyaev, R. M.; Minkin, V. I.; Siebert, M. R.; Tantillo, D. J. Chem. Eur. J. 2010, 16, 2272-2281: "Sandwich Compounds of Transition Metals with Cyclopolyenes and Isolobal Boron Analogues"
Davis, R. L.; Lodewyk, M. W.; Siebert, M. R.; Tantillo, D. J. J. Organomet. Chem. 2013, 748, 68-74: "Complex Consequences: Substituent Effects on Metal - - - Arylmethylium Interactions," part of special issue on "Theory and Mechanistic Studies in Metal-Organic Chemistry."
Dean J. Tantillo, Joseph C. Walsh, Craig A. Merlic, and K. N. Houk: "Chemical Hermaphroditism: The Effect of Cr(CO)3-Complexation on the Structures and Stabilities of Benzylic Reactive Intermediates" Lecture presented at the 217th ACS National Meeting, Anaheim, CA, March 21-25, 1999; paper ORGN 522.
Bruce Hietbrink, Dean J. Tantillo, Craig Merlic, and K. N. Houk: "Interaction of Transition Metals with Non-Classical Carbocations" Poster presented at the 219th ACS National Meeting, San Francisco, CA, March 26-30, 2000; paper ORGN 418.